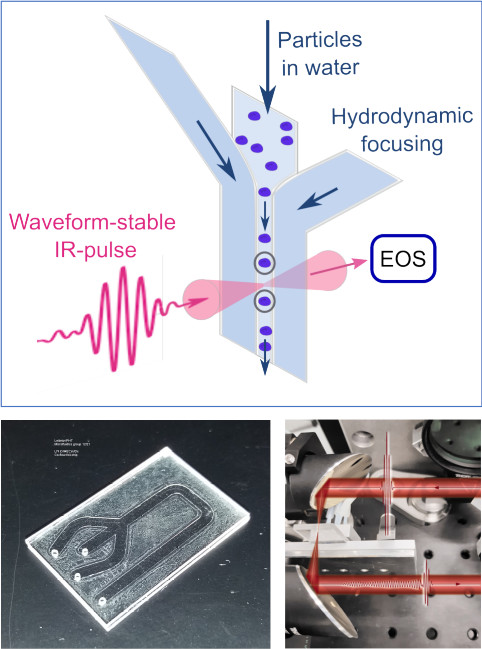
Flow cytometry stands out as a robust tool for investigating individual cells and particles on a large scale, yielding essential insights into biological phenomena. Its effectiveness in swiftly extracting biologically relevant information is largely attributed to the integration of fluorescent labels, a sensitive and selective approach. Despite the strides made, there is a growing demand for label-free methods due to the limitations associated with fluorescent labels, including their occasional unavailability, potential toxicity, and considerable costs. Addressing these challenges, various endeavors have been undertaken to explore label-free alternatives, with vibrational spectroscopy emerging as a particularly promising candidate [1,2]. This technique offers the benefits of minimal sample preparation, non-destructive acquisition of chemically-specific signals, and the potential for ultra-fast data acquisition. The use of infrared (IR) spectroscopy for such applications has been hindered by the strong IR absorption of liquid water and the limited brilliance of contemporary radiation sources.

However, the advent of field-resolved IR spectroscopy (FRS) [3], leveraging brilliant, waveform-controlled femtosecond-laser-based IR sources and field-sensitive detection approaching ultimate sensitivity [4], presents a breakthrough in overcoming these barriers. The integration of a field-resolved spectrometer with rapid spectral acquisition at 38 kHz [5] and an infrared-compatible microfluidic chip has enabled the first-ever demonstration of label-free high-speed IR measurements of particles in flow, achieving a throughput of up to 100 particles per second.

The recorded infrared spectra carry information on the molecular composition of samples, allow for the extraction of properties such as the particle type. Utilizing density-based spatial clustering combined with t-distributed stochastic neighbor embedding reveals the capability to identify different particle types, such as PMMA and PS particles of varying diameters, as well as yeast and THP-1 cells, solely based on spectral information without the need for additional labels.
FRS bears significant potential in realizing label-free, infrared-based flow cytometry and sorting of human cells. Already, diverse particle types can be identified at the individual level through their vibrational spectra, with acquisition times as short as 1 ms per cell. Future applications stand to benefit from the wider bandwidth and increased sensitivity of next-generation spectrometers, facilitating the retrieval of more molecular information and enhancing throughput. This innovative technique holds promise for expanding the scope of vibrational fingerprinting in biological systems and enabling high-throughput screening for low-abundance circulating tumor cells [1].
[1] I. W. Schie, J. Rüger, A. S. Mondol, A. Ramoji, U. Neugebauer, C. Krafft, J. Popp, “High-Throughput Screening Raman Spectroscopy Platform for Label-Free Cellomics”, Anal. Chem. 90, 3 (2018).
[2] J. G. de Pablo, M. Lindley, K. Hiramatsu, K. Goda, “High-Throughput Raman Flow Cytometry and beyond”, Res. Acc. Chem. Res. 54, 9 (2021).
[3] I. Pupeza, M. Huber, M. Trubetskov, W. Schweinberger, S.A. Hussain, C. Hofer, K. Fritsch, M. Poetzlberger, L. Vamos, E. Fill, T. Amotchkina, K.V. Kepesidis, A. Apolonski, N. Karpowicz, V. Pervak, O. Pronin, F. Fleischmann, A. Azzeer, M. Zigman, F. Krausz, “Field-resolved infrared spectroscopy of biological systems”, Nature 577, 52 (2020).
[4] C. Hofer, D. Gerz, M. Högner, T.P. Butler, C. Gaida, T. Heuermann, M. Gebhardt, N. Karpowicz, J. Limpert, F. Krausz, I. Pupeza, “Mid-infrared electric field sampling approaching single-photon sensitivity”, EPJ Web of Conf. 243, 16001 (2020).
[5] A. Weigel, P. Jacob, D. Gröters, T. Buberl, M. Huber, M. Trubetskov, J. Heberle, I. Pupeza, “Ultra-rapid electro-optic sampling of octave-spanning mid-infrared waveforms”, Optics Express 29, 13 (2021).
